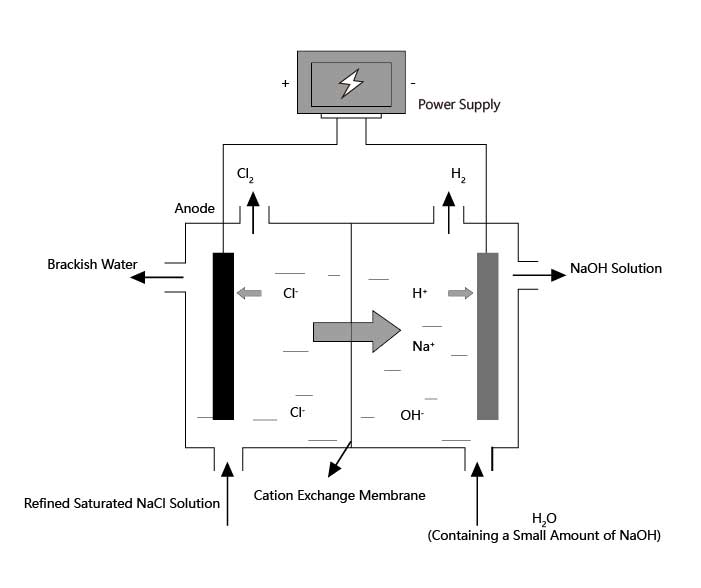
Industrial production of NaOH, Cl2, and H2 utilizes electrolysis of saturated NaCl solution, which is called the Chlor-alkali industry. Chlor-alkali industry is one of the most basic chemical industries, whose products are widely used in light industry, textile, metallurgy, petrochemical, public utilities, and other fields.
Dimensionally stable anodes (DSA) have gradually replaced graphite anodes in the Chlor-alkali industry due to their excellent electrochemical performance. Titanium is considered an optimum choice for electrode substrate, as titanium could spontaneously form a protective oxide layer that protects the substrate from electrolyte erosion. In addition, titanium has no significant resistance to current flow. Regarding the electrocatalytic material, the selection of ruthenium oxide helps to improve the stability of the coating, and titanium oxide is added as a stabilizer for the metal oxide.
Solution Reaction: 2NaCl+2H₂O→2NaOH+Cl₂↑+H₂↑